Question
Which of the following is not correct about xenon hexafluoride?
A.
It has oxidation state of + 6.
B.
The hybridisation involved in $$Xe{F_6}$$ is $$s{p^3}{d^3}.$$
C.
The shape of $$Xe{F_6}$$ is distorted octahedral and can be represented as 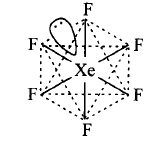

D.
On hydrolysis it gives $$Xe, HF$$ and $${O_2}.$$
Answer :
On hydrolysis it gives $$Xe, HF$$ and $${O_2}.$$
Solution :
On hydrolysis the products formed are $$Xe{O_3}$$ and $$HF.$$
$$Xe{F_6} + 3{H_2}O \to Xe{O_3} + 6HF$$
On hydrolysis the products formed are $$Xe{O_3}$$ and $$HF.$$
$$Xe{F_6} + 3{H_2}O \to Xe{O_3} + 6HF$$