Question
Which of the following is least reactive in a nucleophilic substitution reaction?
A.
$${\left( {C{H_3}} \right)_3}C - Cl$$
B.
$$C{H_2} = CHCl$$
C.
$$C{H_3}C{H_2}Cl$$
D.
$$C{H_2} = CHC{H_2}Cl$$
Answer :
$$C{H_2} = CHCl$$
Solution :
Chlorine of vinyl chloride $$\left( {C{H_2} = CHCl} \right)$$ is non-reactive ( less reactive ) towards nucleophile ( in nucleophilic substitution reaction ) because it shows the following resonating structure due to $$+M$$ - effect of $$-Cl$$ - $$atom.$$
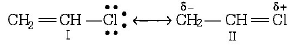
In structure II, $$Cl$$ - $$atom$$ have positive charge and partial double bond character with $$C$$ of vinyl group, so it is more tightly attracted towards the nucleus and does not get replaced by nucleophile in $${S_N}$$ reaction.
Chlorine of vinyl chloride $$\left( {C{H_2} = CHCl} \right)$$ is non-reactive ( less reactive ) towards nucleophile ( in nucleophilic substitution reaction ) because it shows the following resonating structure due to $$+M$$ - effect of $$-Cl$$ - $$atom.$$
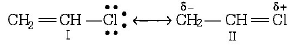
In structure II, $$Cl$$ - $$atom$$ have positive charge and partial double bond character with $$C$$ of vinyl group, so it is more tightly attracted towards the nucleus and does not get replaced by nucleophile in $${S_N}$$ reaction.