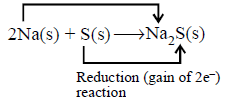Question
Which of the following is correct code for $$x$$ and $$y$$ in the following reaction.
Which of the following is correct code for $$x$$ and $$y$$ in the following reaction.
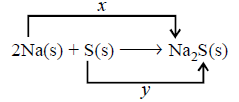
(i) $$x =$$ oxidation reaction, $$y =$$ reduction reaction
(ii) $$x =$$ gain of two electrons, $$y =$$ loss of two electrons,
(iii) $$x =$$ reduction reaction, $$y =$$ oxidation reaction
(iv) $$x =$$ loss of two electrons, $$y =$$ gain of two electrons
A.
(i) and (ii)
B.
(i) and (iv)
C.
(ii) and (iii)
D.
(iii) and (iv)
Answer :
(i) and (iv)
Solution :
Oxidation reaction ( loss of $$2{e^ - })$$
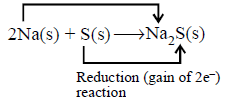
Oxidation reaction ( loss of $$2{e^ - })$$