Question
Which of the following complex ions is expected to absorb visible light ?
Which of the following complex ions is expected to absorb visible light ?
$$\left( {{\text{At}}{\text{. no}}{\text{. of}}\,\,Zn = 30,Sc = 21,} \right.$$ $$\left. {Ti = 22,Cr = 24} \right)$$
A.
$${\left[ {Sc{{\left( {{H_2}O} \right)}_3}{{\left( {N{H_3}} \right)}_3}} \right]^{3 + }}$$
B.
$${\left[ {Ti{{\left( {en} \right)}_2}{{\left( {N{H_3}} \right)}_2}} \right]^{4 + }}$$
C.
$${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
D.
$${\left[ {Zn{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
Solution :
In $${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }},Cr$$ is present as $$C{r^{3 + }}.$$
$$C{r^{3 + }} = \left[ {Ar} \right]3{d^3},4{s^0}$$
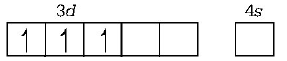
$${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }} = \left[ {Ar} \right]3{d^3}$$

Since, this complex has three unpaired electrons, excitation of electrons is possible and thus, it is expected that this complex will absorb visible light.
In $${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }},Cr$$ is present as $$C{r^{3 + }}.$$
$$C{r^{3 + }} = \left[ {Ar} \right]3{d^3},4{s^0}$$

$${\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }} = \left[ {Ar} \right]3{d^3}$$

Since, this complex has three unpaired electrons, excitation of electrons is possible and thus, it is expected that this complex will absorb visible light.