Question
Which of the following are Lewis acids ?
A.
$$P{H_3}\,{\text{and}}\,BC{l_3}$$
B.
$$AlC{l_3}\,{\text{and}}\,SiC{l_4}$$
C.
$$P{H_3}\,{\text{and}}\,SiC{l_4}$$
D.
$$BC{l_3}\,{\text{and}}\,AlC{l_3}$$
Answer :
$$AlC{l_3}\,{\text{and}}\,SiC{l_4}$$
Solution :
$$BC{l_3}\,{\text{and}}\,AlC{l_3},$$ both have vacant $$p-$$orbital and incomplete octet thus they behave as Lewis acids.
$$SiC{l_4}$$ can accept lone pair of electron in d-orbital of silicon hence it can act as Lewis acid.
Although the most suitable answer is (c). However, both options (c) and (a) can be considered as correct answers.
e.g. hydrolysis of $$SiC{l_4}$$
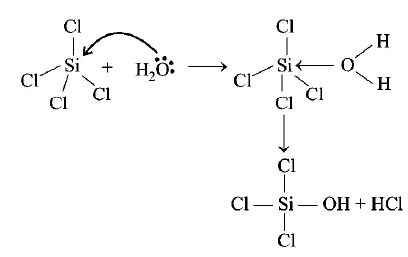
i.e., option (a) $$AlC{l_3}\,{\text{and}}\,SiC{l_4}$$ is also correct.
$$BC{l_3}\,{\text{and}}\,AlC{l_3},$$ both have vacant $$p-$$orbital and incomplete octet thus they behave as Lewis acids.
$$SiC{l_4}$$ can accept lone pair of electron in d-orbital of silicon hence it can act as Lewis acid.
Although the most suitable answer is (c). However, both options (c) and (a) can be considered as correct answers.
e.g. hydrolysis of $$SiC{l_4}$$

i.e., option (a) $$AlC{l_3}\,{\text{and}}\,SiC{l_4}$$ is also correct.