Question
Which amongst the following is the most stable carbocation?
A.
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{+}{\mathop{-C-}}}\,H\]
B.
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-{{C}^{+}}}}}\,\]
C.
\[\overset{+\,\,\,\,\,}{\mathop{C{{H}_{3}}}}\,\]
D.
\[\overset{\,\,\,\,\,+}{\mathop{C{{H}_{3}}C{{H}_{2}}}}\,\]
Answer :
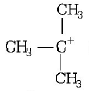
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-{{C}^{+}}}}}\,\]
Solution :
The most stable carbocation is $$t-alkyl$$ carbocation. The order of stability of $$alkyl$$ carbocation is $$ter - alkyl > \sec - alkyl > pri - alkyl > CH_3^ + $$ carbocation.
This stability order is described with the help of hyperconjugation and inductive effect. On the basis of hyperconjugation, $${\left( {C{H_3}} \right)_2}\mathop C\limits^ + H$$ shows six resonating structures due to the presence of six $$\alpha {\text{ - }}C - H$$ bonds,
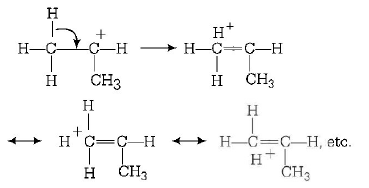
 shows nine resonating structures due to the presence of nine $$\alpha {\text{ - }}C - H$$ bonds.
shows nine resonating structures due to the presence of nine $$\alpha {\text{ - }}C - H$$ bonds.
Greater the $$\alpha \,H - atom$$ greater will be the hyper conjugation resonating structure and therefore, greater will be the stability.
$$\mathop C\limits^ + {H_3}$$ does not show the property of resonance while $$C{H_3} - \mathop C\limits^ + {H_2}$$ shows three resonating strictures due to presence of three $$\alpha {\text{ - }}C - H$$ bonds. Hence, larger number of resonating structures are possible in (B), so it is most stable. The above order of stability is also explained with the help of $$(+)$$ $$I$$ - effect of $$ - C{H_3}$$ group. More the number of $$ - C{H_3}$$ group more will be tendency to displace the electrons towards positively charged carbon of carbocation. Thus, positive charge is decreased or compensated and stability of carbocation is increased.
The most stable carbocation is $$t-alkyl$$ carbocation. The order of stability of $$alkyl$$ carbocation is $$ter - alkyl > \sec - alkyl > pri - alkyl > CH_3^ + $$ carbocation.
This stability order is described with the help of hyperconjugation and inductive effect. On the basis of hyperconjugation, $${\left( {C{H_3}} \right)_2}\mathop C\limits^ + H$$ shows six resonating structures due to the presence of six $$\alpha {\text{ - }}C - H$$ bonds,

 shows nine resonating structures due to the presence of nine $$\alpha {\text{ - }}C - H$$ bonds.
shows nine resonating structures due to the presence of nine $$\alpha {\text{ - }}C - H$$ bonds.Greater the $$\alpha \,H - atom$$ greater will be the hyper conjugation resonating structure and therefore, greater will be the stability.
$$\mathop C\limits^ + {H_3}$$ does not show the property of resonance while $$C{H_3} - \mathop C\limits^ + {H_2}$$ shows three resonating strictures due to presence of three $$\alpha {\text{ - }}C - H$$ bonds. Hence, larger number of resonating structures are possible in (B), so it is most stable. The above order of stability is also explained with the help of $$(+)$$ $$I$$ - effect of $$ - C{H_3}$$ group. More the number of $$ - C{H_3}$$ group more will be tendency to displace the electrons towards positively charged carbon of carbocation. Thus, positive charge is decreased or compensated and stability of carbocation is increased.