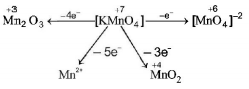
Question
When $$KMn{O_4}$$ acts as an oxidising agent and ultimately forms $${\left[ {Mn{O_4}} \right]^{ - 2}},Mn{O_2},M{n_2}{O_3},M{n^{ + 2}}$$ then the number of electrons transfered in each case respectively is
A.
$$4,3,1,5$$
B.
$$1,5,3,7$$
C.
$$1,3,4,5$$
D.
$$3,5,7,1.$$
Answer :
$$1,3,4,5$$
Solution :