Question
When $$C{l_2}$$ gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from
A.
zero to + 1 and zero to - 5
B.
zero to - 1 and zero to + 5
C.
zero to - 1 and zero to + 3
D.
zero to + 1 and zero to - 3
Answer :
zero to - 1 and zero to + 5
Solution :
When chlorine gas reacts with hot and concentrated $$NaOH$$ solution, it disproportionates into chloride $$\left( {C{l^ - }} \right)$$ and chlorate $$\left( {ClO_3^ - } \right)$$ ions.
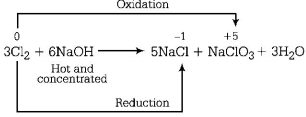
In this process, oxidation number of chlorine changes from 0 to - 1 and 0 to + 5.
NOTE
In disproportionation reactions, the same element undergoes oxidation as well as reduction.
When chlorine gas reacts with hot and concentrated $$NaOH$$ solution, it disproportionates into chloride $$\left( {C{l^ - }} \right)$$ and chlorate $$\left( {ClO_3^ - } \right)$$ ions.

In this process, oxidation number of chlorine changes from 0 to - 1 and 0 to + 5.
NOTE
In disproportionation reactions, the same element undergoes oxidation as well as reduction.