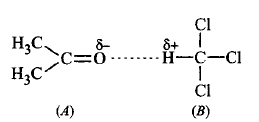Question
When acetone and chloroform are mixed together, which of the following observations is correct ?
When acetone and chloroform are mixed together, which of the following observations is correct ?
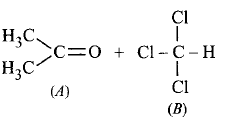
A.
$$A - A$$ and $$B - B$$ interactions are stronger than $$A - B$$ interactions.
B.
$$A - A$$ and $$B - B$$ interactions are weaker than $$A - B$$ interactions.
C.
$$A - A, B - B$$ and $$A - B$$ interactions are equal.
D.
The liquids form separate layers and are immiscible.
Answer :
$$A - A$$ and $$B - B$$ interactions are weaker than $$A - B$$ interactions.
Solution :
When acetone and chloroform are mixed together, a hydrogen bond is formed between them which increases intermolecular interactions. Hence, $$A - B$$ interactions are stronger than $$A - A$$ and $$B - B$$ interactions.
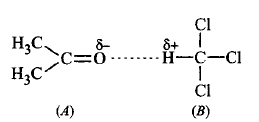
When acetone and chloroform are mixed together, a hydrogen bond is formed between them which increases intermolecular interactions. Hence, $$A - B$$ interactions are stronger than $$A - A$$ and $$B - B$$ interactions.