Question
What will be the difference between electromagnetic radiation shown in $$A$$ and $$B$$ respectively ?
What will be the difference between electromagnetic radiation shown in $$A$$ and $$B$$ respectively ?
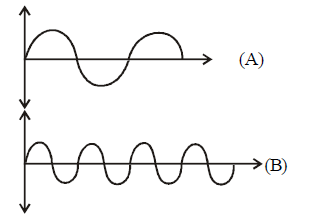
(i) Velocity
(ii) Wavelength
(iii) Frequency
(iv) Energy
A.
(ii) only
B.
(ii) and (iv)
C.
(ii), (iii) and (iv)
D.
(iv) only
Answer :
(ii), (iii) and (iv)
Solution :
$$e/m$$ waves shown in figure $$A$$ has higher wavelength in comparison to $$e/m$$ waves shown in figure $$B.$$
Thus these waves also differ in frequency and energy. $$\nu = \frac{c}{\lambda }$$
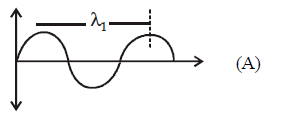
$$ \Rightarrow {E_1} = \frac{{hc}}{{{\lambda _1}}}$$
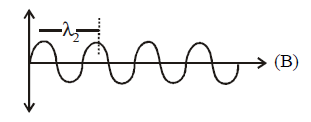
$$ \Rightarrow {E_2} = \frac{{hc}}{{{\lambda _2}}}\,\,\,\,{\lambda _1} > {\lambda _2} \Rightarrow {E_1} < {E_2}$$
$$e/m$$ waves shown in figure $$A$$ has higher wavelength in comparison to $$e/m$$ waves shown in figure $$B.$$
Thus these waves also differ in frequency and energy. $$\nu = \frac{c}{\lambda }$$

$$ \Rightarrow {E_1} = \frac{{hc}}{{{\lambda _1}}}$$

$$ \Rightarrow {E_2} = \frac{{hc}}{{{\lambda _2}}}\,\,\,\,{\lambda _1} > {\lambda _2} \Rightarrow {E_1} < {E_2}$$