Question
What is $$Z$$ in the following sequence of reactions ?
What is $$Z$$ in the following sequence of reactions ?
\[Z\xrightarrow{PC{{l}_{5}}}X\xrightarrow{Alc.\,KOH}Y\] \[\xrightarrow[\left( \text{ii} \right)\,{{H}_{2}}O;\,\text{boil}]{\left( \text{i} \right)\,Conc.\,{{H}_{2}}S{{O}_{4}}}Z\]
A.
$$C{H_3}C{H_2}C{H_2}OH$$
B.
$$C{H_3}CH\left( {OH} \right)C{H_3}$$
C.
$${\left( {C{H_3}C{H_2}} \right)_2}CHOH$$
D.
$$C{H_3}CH = C{H_2}$$
Answer :
$$C{H_3}CH\left( {OH} \right)C{H_3}$$
Solution :
Reagents used in the various steps indicate that the compound $$Z$$ has an alcoholic group. This set of reactions is possible only when $$Z$$ is $$C{H_3}CHOHC{H_3}.$$
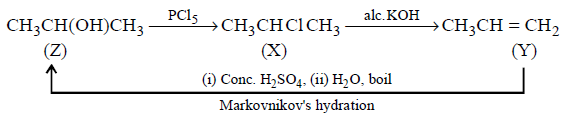
In options A and C, $$Y$$ cannot be converted back into D by the given series of reactions.
Reagents used in the various steps indicate that the compound $$Z$$ has an alcoholic group. This set of reactions is possible only when $$Z$$ is $$C{H_3}CHOHC{H_3}.$$
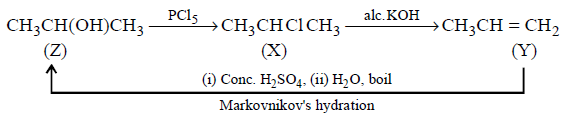
In options A and C, $$Y$$ cannot be converted back into D by the given series of reactions.