Question
What is the structure of the major product when phenol is treated with bromine water ?
A.


B.
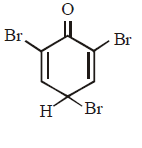

C.


D.


Answer :


Solution :
Phenol has activating ( electron releasing ) $$–OH$$ group and bromine water supplies $$B{r^ + }\,ion$$ easily, hence under such conditions reaction does not stop at monobromo or dibromo stage but a fully brominated ( 2, 4, 6, - tribromophenol ) compound is the final product.

Phenol has activating ( electron releasing ) $$–OH$$ group and bromine water supplies $$B{r^ + }\,ion$$ easily, hence under such conditions reaction does not stop at monobromo or dibromo stage but a fully brominated ( 2, 4, 6, - tribromophenol ) compound is the final product.
