Question
What is the formal charge on carbon atom in the following two structures :
What is the formal charge on carbon atom in the following two structures :

A.
0, - 2
B.
0, 0
C.
+ 2, -2
D.
+ 1, - 1
Answer :
0, 0
Solution :
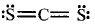
Formal charge on $$C$$ atom = Valence electrons - Lone pair of electrons $$ - {1 \over 2}$$ × Bonding electron
$$\eqalign{ & = 4 - 0 - {1 \over 2} \times 8 \cr & = 0 \cr} $$
Similarly, formal charge on $$C$$ atom in other structure = 0
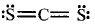
Formal charge on $$C$$ atom = Valence electrons - Lone pair of electrons $$ - {1 \over 2}$$ × Bonding electron
$$\eqalign{ & = 4 - 0 - {1 \over 2} \times 8 \cr & = 0 \cr} $$
Similarly, formal charge on $$C$$ atom in other structure = 0