Question
What is the dominant intermolecular force on bond that must be overcome in converting liquid $$C{H_3}OH$$ to a gas?
A.
Hydrogen bonding
B.
Dipole-dipole interaction
C.
Covalent bonds
D.
London or dispersion force
Answer :
Hydrogen bonding
Solution :
In between $$C{H_3}OH$$ molecules intermolecular $$H - {\text{bonding}}$$ exist.
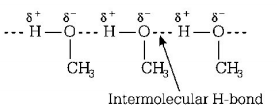
Hence, it is the intermolecular $$H - {\text{bonding}}$$ that must be overcome in converting liquid $$C{H_3}OH$$ to gas.
In between $$C{H_3}OH$$ molecules intermolecular $$H - {\text{bonding}}$$ exist.

Hence, it is the intermolecular $$H - {\text{bonding}}$$ that must be overcome in converting liquid $$C{H_3}OH$$ to gas.