Question
What is the correct order of decreasing stability of the following cations?
What is the correct order of decreasing stability of the following cations?
$$\eqalign{
& \mathop {C{H_3} - \mathop C\limits^ + H - C{H_3}}\limits_{\left( {\text{I}} \right)} \cr
& \mathop {C{H_3} - \mathop C\limits^ + H - OC{H_3}}\limits_{\left( {{\text{II}}} \right)} \cr
& \mathop {C{H_3} - \mathop C\limits^ + H - C{H_2} - OC{H_3}}\limits_{\left( {{\text{III}}} \right)} \cr} $$
A.
(II) > (I) > (III)
B.
(II) > (III) > (I)
C.
(III) > (I) > (II)
D.
(I) > (II) > (III)
Answer :
(II) > (I) > (III)
Solution :

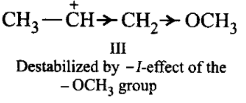
Thus, the stability of carbocations decreases in the order : II > I > III.

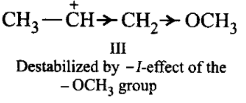
Thus, the stability of carbocations decreases in the order : II > I > III.