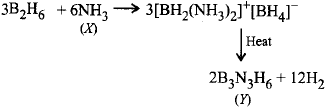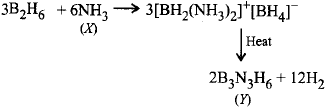Question
What are $$X$$ and $$Y$$ in the reaction?
What are $$X$$ and $$Y$$ in the reaction?
$$3{B_2}{H_6} + 6X \to 3{\left[ {B{H_2}{{\left( X \right)}_2}} \right]^ + }$$ \[{{\left[ B{{H}_{4}} \right]}^{-}}\xrightarrow{\text{heat}}Y+12{{H}_{2}}\]
A.
$$X = N{H_3},Y = {B_3}{N_3}{H_6}$$
B.
$$X = CO,Y = B{H_3}CO$$
C.
$$X = NaH,Y = NaF$$
D.
$$X = N{F_3},Y = {B_3}{N_3}$$
Answer :
$$X = N{H_3},Y = {B_3}{N_3}{H_6}$$
Solution :