Question
Though covalent in nature, methanol is soluble in water, why?
A.
Methanol is transparent like water.
B.
Due to hydrogen bonding between methanol and water molecules.
C.
Due to van der Waals' forces between methanol and water.
D.
Due to covalent attraction forces.
Answer :
Due to hydrogen bonding between methanol and water molecules.
Solution :
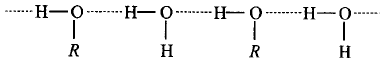
In methanol, $$R$$ is $$ - C{H_3}$$ group.
Hydrogen bonding between methanol and water.
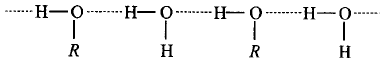
In methanol, $$R$$ is $$ - C{H_3}$$ group.
Hydrogen bonding between methanol and water.