Question
The temperature dependence of the rate of a chemical reaction is given by Arrhenius equation, $$k = A{e^{ - \frac{{Ea}}{{RT}}}}.$$ Which of the following graphs will be a straight line?
A.
$$\ln \,A\,\,vs\,\,\frac{1}{T}$$
B.
$$\ln \,A\,\,vs\,\,{E_a}$$
C.
$$\ln \,k\,\,vs\,\,\frac{1}{T}$$
D.
$$\ln \,k\,\,vs\,\, - \frac{{{E_a}}}{R}$$
Answer :
$$\ln \,k\,\,vs\,\,\frac{1}{T}$$
Solution :
The plot of $$\ln \,k\,\,vs\,\,\frac{1}{T}$$ gives a straight line according to the equation, $$\ln k = - \frac{{{E_a}}}{{RT}} + \ln A$$
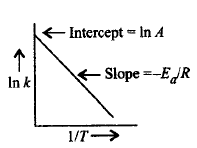
The plot of $$\ln \,k\,\,vs\,\,\frac{1}{T}$$ gives a straight line according to the equation, $$\ln k = - \frac{{{E_a}}}{{RT}} + \ln A$$
