Question
The stability of carbanions in the following compounds,
The stability of carbanions in the following compounds,
$$\left( {\text{i}} \right)RCH = \bar CH$$
$$\left( {{\text{ii}}} \right)$$ 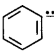
$$\left( {{\text{iii}}} \right){R_2}C = \bar CH$$
$$\left( {{\text{iv}}} \right){R_3}C - \bar C{H_2}$$
is in the order of
A.
(iv) > (ii) > (iii) > (i)
B.
(i) > (iii) > (ii) > (iv)
C.
(i) > (ii) > (iii) > (iv)
D.
(ii) > (iii) > (iv) > (i)
Answer :
(i) > (ii) > (iii) > (iv)
Solution :
Higher the no. of electron releasing groups lower will be stability of carbanion, and vice versa. So, the order of stability of carbanions is (i) > (ii) > (iii) > (iv).
Higher the no. of electron releasing groups lower will be stability of carbanion, and vice versa. So, the order of stability of carbanions is (i) > (ii) > (iii) > (iv).