Question
The stability of carbanions in the following
The stability of carbanions in the following
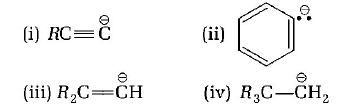
is in the order of
A.
(i) > (ii) > (iii) > (iv)
B.
(ii) > (iii) > (iv) > (i)
C.
(iv) > (ii) > (iii) > (i)
D.
(i) > (iii) > (ii) > (iv)
Answer :
(i) > (ii) > (iii) > (iv)
Solution :
The carbanion with more $$s$$ - character is more stable. Thus, the order of stability is
$$RC \equiv \mathop C\limits^\Theta > {C_6}H_5^\Theta > {R_2}C = \mathop C\limits^\Theta H > {R_3}C - \mathop C\limits^\Theta {H_2}$$
The carbanion with more $$s$$ - character is more stable. Thus, the order of stability is
$$RC \equiv \mathop C\limits^\Theta > {C_6}H_5^\Theta > {R_2}C = \mathop C\limits^\Theta H > {R_3}C - \mathop C\limits^\Theta {H_2}$$