Question
The species, having bond angles of $${120^ \circ }$$ is
A.
$$P{H_3}$$
B.
$$Cl{F_3}$$
C.
$$NC{l_3}$$
D.
$$BC{l_3}$$
Answer :
$$BC{l_3}$$
Solution :
The species having bond angles of $${120^ \circ }$$ is $$BC{l_3}.$$
It is $$s{p^2}$$-hybridised and central atom does not have any lone pair of electrons.
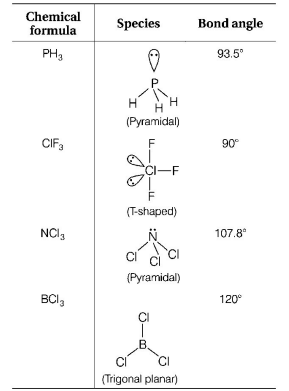
The species having bond angles of $${120^ \circ }$$ is $$BC{l_3}.$$
It is $$s{p^2}$$-hybridised and central atom does not have any lone pair of electrons.
