Question
The shape of water molecule which should be tetrahedral has a bent or distorted tetrahedral shape with a bond angle $${104.5^ \circ }.$$ What could be the reason for this?
A.
$$lp{\text{ - }}lp$$ repulsion is more than $$lp{\text{ - }}bp$$ repulsion.
B.
$$lp{\text{ - }}bp$$ repulsion is more than $$lp{\text{ - }}lp$$ repulsion.
C.
$$lp{\text{ - }}lp$$ repulsion is equal to $$lp{\text{ - }}bp$$ repulsion.
D.
Presence of lone pair does not affect the bond angle.
Answer :
$$lp{\text{ - }}lp$$ repulsion is more than $$lp{\text{ - }}bp$$ repulsion.
Solution :
Due to presence of lone pairs, the repulsion is more which changes the bond angle to $${104.5^ \circ }.$$
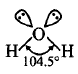
Due to presence of lone pairs, the repulsion is more which changes the bond angle to $${104.5^ \circ }.$$
