Question
The reaction of toluene with $$C{l_2}$$ in the presence of $$FeC{l_3}$$ gives $$'X'$$ and reaction in presence of light gives $$'Y’.$$ Thus, $$'X’$$ and $$'Y’$$ are
A.
$$X=$$ benzal chloride, $$Y = $$ $$o$$ - chlorotoluene
B.
$$X =$$ $$m$$ - chlorotoluene, $$Y =$$ $$p$$ - chlorotoluene
C.
$$X =o$$ and $$p$$ - chlorotoluene, $$Y =$$ trichloromethyl benzene
D.
$$X =$$ benzyl chloride, $$Y =$$ $$m$$ - chlorotoluene
Answer :
$$X =o$$ and $$p$$ - chlorotoluene, $$Y =$$ trichloromethyl benzene
Solution :
Key Idea In the presence of halogen carrier, electrophilic substitution occurs while in the presence of sunlight, substitution, occurs at the side chain.
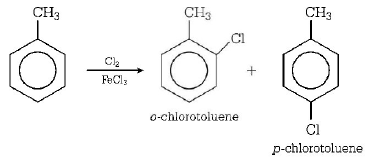
$$FeC{l_3} + C{l_2} \to FeCl_4^ - + C{l^ + }$$
Electrophile attacking species
( $$\because \,\, - C{H_3}$$ is an $$o/p$$ - directing group. )
In presence of $$hv,$$ reaction is free radical substitution reaction.

Key Idea In the presence of halogen carrier, electrophilic substitution occurs while in the presence of sunlight, substitution, occurs at the side chain.

$$FeC{l_3} + C{l_2} \to FeCl_4^ - + C{l^ + }$$
Electrophile attacking species
( $$\because \,\, - C{H_3}$$ is an $$o/p$$ - directing group. )
In presence of $$hv,$$ reaction is free radical substitution reaction.
