Question
The order of stability of the following tautomeric compound is
The order of stability of the following tautomeric compound is
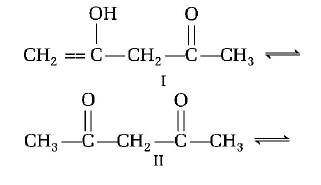
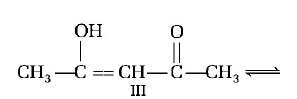
A.
I > II > III
B.
III > II > I
C.
II > I > III
D.
II > III > I
Answer :
III > II > I
Solution :
The enols of $$\beta - dicarbonyl$$ compounds are more stable because of conjugation and intramolecular $$H-$$ bonding. Thus, the order of stability is
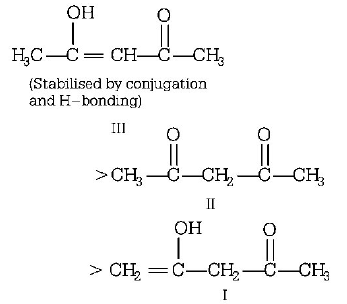
Less stable as $$\left( = \right)$$ bond
is not in conjugation with carbonyl group
The enols of $$\beta - dicarbonyl$$ compounds are more stable because of conjugation and intramolecular $$H-$$ bonding. Thus, the order of stability is

Less stable as $$\left( = \right)$$ bond
is not in conjugation with carbonyl group