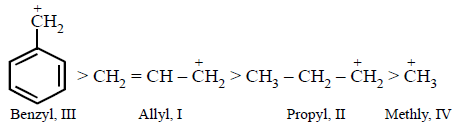Question
The order of stability of the following carbocations :
The order of stability of the following carbocations :
$$\eqalign{
& {\text{(I)}}C{H_2} = CH - \mathop C\limits^ + {H_2} \cr
& {\text{(II)}}C{H_3} - C{H_2} - \mathop C\limits^ + {H_2} \cr} $$
$${\text{(III)}}$$ 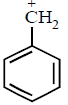
$${\text{(IV)}}\mathop C\limits^ + {H_3}\,{\text{is}}\,{\text{:}}$$
A.
IV > III > II > I
B.
II > III > I > IV
C.
III > I > II > IV
D.
III > I > IV > II
Answer :
III > I > II > IV
Solution :
Higher stability of allyl and aryl substituted methyl carbocation is due to dispersal of positive charge due to resonance
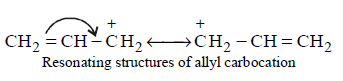
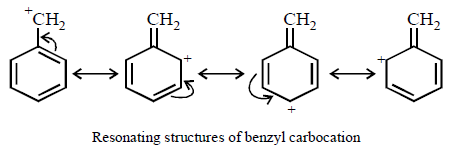
Hence the correct order of stability will be
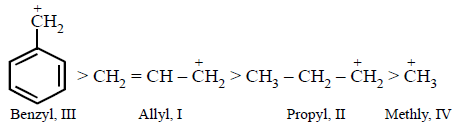
Higher stability of allyl and aryl substituted methyl carbocation is due to dispersal of positive charge due to resonance
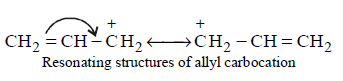

Hence the correct order of stability will be