Question
The order of reactivity of the following alcohols towards $$conc.$$ $$HCl$$ is
The order of reactivity of the following alcohols towards $$conc.$$ $$HCl$$ is
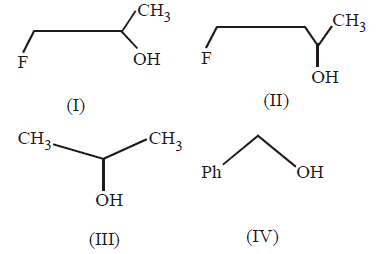
A.
I > II > III > IV
B.
I > III > II > V
C.
IV > III > II > I
D.
IV > III > I > II
Answer :
IV > III > II > I
Solution :
The order of reactivity depends upon the stability of the carbocations formed.
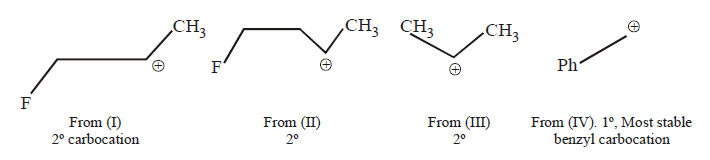
Remember that presence of electron-withdrawing group intensifies i.e., destabilises the carbocation thus (i) and (ii) are less stable than (iii). Further (i); is less stable than (ii) because $$–I$$ effect is more pronounced in (i) due to less distance between $$F$$ and positive charge. Thus the stability order of the four carbocations and reactivity of their parent alcohols will be IV > III > II > I
The order of reactivity depends upon the stability of the carbocations formed.

Remember that presence of electron-withdrawing group intensifies i.e., destabilises the carbocation thus (i) and (ii) are less stable than (iii). Further (i); is less stable than (ii) because $$–I$$ effect is more pronounced in (i) due to less distance between $$F$$ and positive charge. Thus the stability order of the four carbocations and reactivity of their parent alcohols will be IV > III > II > I