Question
The molecules $$B{F_3}$$ and $$N{F_3}$$ are both covalent compounds, but $$B{F_3}$$ is non polar whereas $$N{F_3}$$ is polar. The reason for this is
A.
atomic size of boron is larger than nitrogen
B.
Boron is metal while nitrogen is gas
C.
$$B - F$$ bonds are non-polar while $$N - F$$ bonds are polar
D.
$$B{F_3}$$ is planar but $$N{F_3}$$ is pyramidal
Answer :
$$B{F_3}$$ is planar but $$N{F_3}$$ is pyramidal
Solution :
The shape of $$B{F_3}$$ is trigonal planar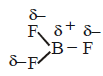 and $$\mu = 0$$ hence it is non polar.
and $$\mu = 0$$ hence it is non polar.
The shape of $$N{F_3}$$ is pyramidal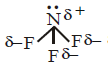 and $$\mu \ne 0$$ hence it is polar.
and $$\mu \ne 0$$ hence it is polar.
The shape of $$B{F_3}$$ is trigonal planar
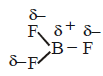 and $$\mu = 0$$ hence it is non polar.
and $$\mu = 0$$ hence it is non polar. The shape of $$N{F_3}$$ is pyramidal
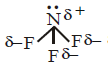 and $$\mu \ne 0$$ hence it is polar.
and $$\mu \ne 0$$ hence it is polar.