Question
The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds to :
A.
ionization of benzoic acid.
B.
dimerization of benzoic acid.
C.
trimerization of benzoic acid.
D.
solvation of benzoic acid.
Answer :
dimerization of benzoic acid.
Solution :
Benzoic acid exists as dimer in benzene.
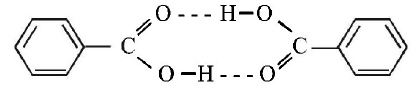
[ Normal molecular mass = 122 amu observed molecular mass = 244 amu, in case of complete association ]
$$\alpha = \frac{{20}}{{100}} = 0.2\,\,\,\alpha = \frac{{20}}{{100}} = 0.2\,\,\,\alpha = \frac{{20}}{{100}} = 0.2$$
Benzoic acid exists as dimer in benzene.
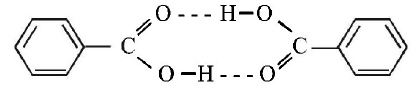
[ Normal molecular mass = 122 amu observed molecular mass = 244 amu, in case of complete association ]
$$\alpha = \frac{{20}}{{100}} = 0.2\,\,\,\alpha = \frac{{20}}{{100}} = 0.2\,\,\,\alpha = \frac{{20}}{{100}} = 0.2$$