Question
The maximum number of $${90^ \circ }$$ angles between bond pair-bond pair of electrons is observed in
A.
$$ds{p^2}$$ hybridization
B.
$$s{p^3}d$$ hybridization
C.
$$ds{p^3}$$ hybridization
D.
$$s{p^3}{d^2}$$ hybridization
Answer :
$$s{p^3}{d^2}$$ hybridization
Solution :
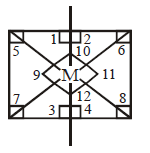
$$s{p^3}{d^2}$$ hybridisation
Number of $${90^ \circ }$$ angle between bonds $$ = 12$$

$$s{p^3}{d^2}$$ hybridisation
Number of $${90^ \circ }$$ angle between bonds $$ = 12$$