Question
The increasing order of $$pKa$$ of the following amino acids in aqueous solution is :
The increasing order of $$pKa$$ of the following amino acids in aqueous solution is :
$$Gly{\text{ }}Asp{\text{ }}Lys{\text{ }}Arg$$
A.
$$Asp < Gly < Arg < Lys$$
B.
$$Gly < Asp < Arg{\text{ }} < {\text{ }}Lys$$
C.
$$Asp < Gly < Lys < Arg$$
D.
$$Arg{\text{ }} < Lys < Gly < Asp$$
Answer :
$$Asp < Gly < Lys < Arg$$
Solution :
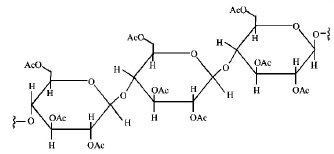
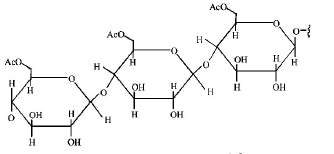
Structure of the given $$\alpha - $$ amino acids are :
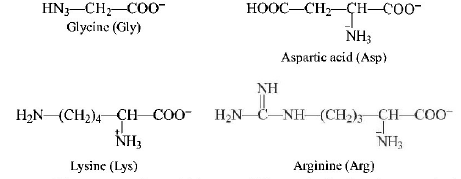
Here, aspartic acid is an acidic and glycine is a neutral amino acid while lysine and arginine are basic amino acids. Also, arginine is more basic due to the stronger basic functional groups.
∴ The order of $$pKa$$ value is directly proportional to the basic strength of amino acids, i.e.
$$Arg{\text{ }} < Lys < Gly < Asp.$$
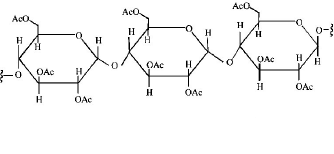
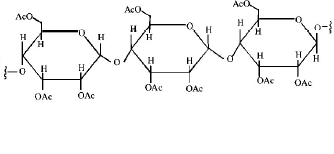
Structure of the given $$\alpha - $$ amino acids are :

Here, aspartic acid is an acidic and glycine is a neutral amino acid while lysine and arginine are basic amino acids. Also, arginine is more basic due to the stronger basic functional groups.
∴ The order of $$pKa$$ value is directly proportional to the basic strength of amino acids, i.e.
$$Arg{\text{ }} < Lys < Gly < Asp.$$