Question
The hybridization of orbitals of $$N$$ atom in $$NO_3^ - ,\,NO_2^ + $$ and $$NH_4^ + $$ are respectively:
A.
$$sp,s{p^2}s{p^3}$$
B.
$$s{p^2},sp,s{p^3}$$
C.
$$sp,s{p^3},s{p^2}$$
D.
$$s{p^2},s{p^3},sp$$
Answer :
$$s{p^2},sp,s{p^3}$$
Solution :
The formula to find the hybridisation of central atom is
$$Z = \frac{1}{2}$$ [Number of valence electrons on central atom $$ + $$ No. of monovalent atom altached to it $$ + $$ negative charge if any $$ - $$ positive charge if any]
For $$N{O_3} - Z = \frac{1}{2}\left[ {5 + 0 + 1 - 0} \right] = 3$$
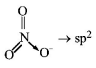
For $$NO_2^ + ,Z = \frac{1}{2}\left[ {5 + 0 + 0 - 1} \right] = 2$$
.PNG)
For $$NH_4^ + ,\,Z = \frac{1}{2}\left[ {5 + 4 + 0 - 1} \right] = 4$$
.PNG)
The formula to find the hybridisation of central atom is
$$Z = \frac{1}{2}$$ [Number of valence electrons on central atom $$ + $$ No. of monovalent atom altached to it $$ + $$ negative charge if any $$ - $$ positive charge if any]
For $$N{O_3} - Z = \frac{1}{2}\left[ {5 + 0 + 1 - 0} \right] = 3$$

For $$NO_2^ + ,Z = \frac{1}{2}\left[ {5 + 0 + 0 - 1} \right] = 2$$
.PNG)
For $$NH_4^ + ,\,Z = \frac{1}{2}\left[ {5 + 4 + 0 - 1} \right] = 4$$
.PNG)