Question
The hybridisations of atomic orbitals of nitrogen in $$NO_2^ + ,NO_3^ - \,{\text{and}}\,NH_4^ + $$ respectively are
A.
$$sp,s{p^3}\,{\text{and}}\,s{p^2}$$
B.
$$s{p^2},s{p^3}\,{\text{and}}\,sp$$
C.
$$sp,s{p^2}\,{\text{and}}\,s{p^3}$$
D.
$$s{p^2},sp\,{\text{and}}\,s{p^3}$$
Answer :
$$sp,s{p^2}\,{\text{and}}\,s{p^3}$$
Solution :
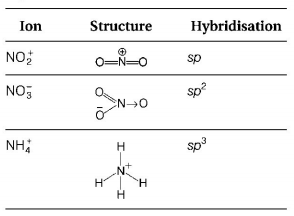
Thus, option (C) is correct.

Thus, option (C) is correct.