Question
The high density of water compared to ice is due to
A.
hydrogen bonding interactions
B.
dipole-dipole interactions
C.
dipole-induced dipole interactions
D.
induced dipole-induced dipole interactions
Answer :
hydrogen bonding interactions
Solution :
Due to polar nature, water molecules show intermolecular hydrogen bonding as
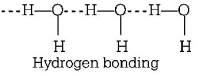
whereas the ice has open structure with large number of vacant spaces. So, density of ice is lower than water.
Due to polar nature, water molecules show intermolecular hydrogen bonding as

whereas the ice has open structure with large number of vacant spaces. So, density of ice is lower than water.