Question
The heating of phenyl-methyl ethers with $$HI$$ produces.
A.
ethyl chlorides
B.
iodobenzene
C.
phenol
D.
benzene
Answer :
phenol
Solution :
Thinking Process This problem is based on the resonance stabilisation.
In anisol, methyl phenyl oxonium ion is formed by protonation of ether. The bond between $$O - C{H_3}$$ is weaker than the bond between $$O - {C_6}{H_5},$$ because the carbon of phenyl group is $$s{p^2}$$ - hybridised and there is a partial double bond character. Thus, the reaction yields phenol and alkyl halide.
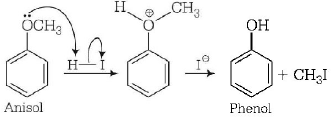
Thinking Process This problem is based on the resonance stabilisation.
In anisol, methyl phenyl oxonium ion is formed by protonation of ether. The bond between $$O - C{H_3}$$ is weaker than the bond between $$O - {C_6}{H_5},$$ because the carbon of phenyl group is $$s{p^2}$$ - hybridised and there is a partial double bond character. Thus, the reaction yields phenol and alkyl halide.
