Question
The heat of atomization of $$P{H_3}\left( g \right)$$ is $$228\,kcal\,mo{l^{ - 1}}$$ and that of $${P_2}{H_4}\left( g \right)$$ is $$335\,kcal\,mo{l^{ - 1}}.$$ The energy of the $$P-P$$ bond is
A.
$$102\,kcal\,mo{l^{ - 1}}$$
B.
$$51\,kcal\,mo{l^{ - 1}}$$
C.
$$26\,kcal\,mo{l^{ - 1}}$$
D.
$$204\,kcal\,mo{l^{ - 1}}$$
Answer :
$$51\,kcal\,mo{l^{ - 1}}$$
Solution :
$$\eqalign{ & {\text{Bond dissociation energy of}} \cr & P{H_3}\left( g \right) = 228\,kcal\,mo{l^{ - 1}} \cr & P - H\,\,{\text{bond energy}} = \frac{{228}}{3} = 76\,kcal\,mo{l^{ - 1}} \cr} $$
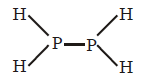
$$\eqalign{ & {\text{Bond energy of}}\,\,4\left( {P - H} \right) + \left( {P - P} \right) \cr & = 355\,kcal\,mo{l^{ - 1}} \cr & {\text{or}}\,\,4 \times 76 + \left( {P - P} \right) = 355\,kcal\,mo{l^{ - 1}} \cr & P - P\,\,{\text{bond energy}} = 51\,kcal\,mo{l^{ - 1}} \cr} $$
$$\eqalign{ & {\text{Bond dissociation energy of}} \cr & P{H_3}\left( g \right) = 228\,kcal\,mo{l^{ - 1}} \cr & P - H\,\,{\text{bond energy}} = \frac{{228}}{3} = 76\,kcal\,mo{l^{ - 1}} \cr} $$
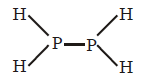
$$\eqalign{ & {\text{Bond energy of}}\,\,4\left( {P - H} \right) + \left( {P - P} \right) \cr & = 355\,kcal\,mo{l^{ - 1}} \cr & {\text{or}}\,\,4 \times 76 + \left( {P - P} \right) = 355\,kcal\,mo{l^{ - 1}} \cr & P - P\,\,{\text{bond energy}} = 51\,kcal\,mo{l^{ - 1}} \cr} $$