Question
The given figure shows the corrosion of iron in atmosphere,
The given figure shows the corrosion of iron in atmosphere,
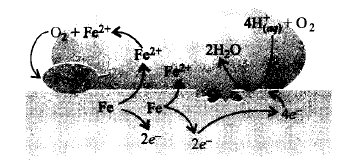
Fill in the blanks by choosing an appropriate option. At a particular spot of an object made of iron, $$\underline {\left( {\text{i}} \right)} $$ of iron to ferrous ion takes place and that spot behaves as $$\underline {\left( {{\text{ii}}} \right)} $$ . Electrons released at anodic spot move through the metal and go to another spot on the metal and reduce oxygen in presence of $${H^ + }.$$ This spot behaves as $$\underline {\left( {{\text{iii}}} \right)} \,$$ . The ferrous ions are further oxidised by atmospheric oxygen to ferric ions which come out as rust, $$\underline {\left( {{\text{iv}}} \right)} $$ and with further production of $$\underline {\left( {\text{v}} \right)} $$ ions.
| (i) | (ii) | (iii) | (iv) | (v) | |
|---|---|---|---|---|---|
| (a) | oxidation | anode | cathode | Fe2O3.xH2O | hydrogen |
| (b) | reduction | cathode | anode | Fe3O4 | hydroxide |
| (c) | oxidation | cathode | anode | Fe2O3.xH2O | hydrogen |
| (d) | oxidation | anode | cathode | Fe2O3.H2O | ferrous |
A.
(a)
B.
(b)
C.
(c)
D.
(d)
Answer :
(a)