Question
The following two figures represent
The following two figures represent
A.
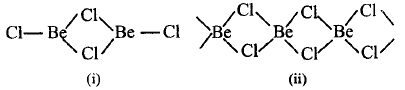
$$\left( {\text{i}} \right)BeC{l_2}$$ is a dimer in vapour phase ; $$\left( {{\text{ii}}} \right)BeC{l_2}$$ is chain structure in solid state
B.
$$\left( {\text{i}} \right)BeC{l_2}$$ is in solid state ; $$\left( {{\text{ii}}} \right)BeC{l_2}$$ is in vapour phase
C.
$$\left( {\text{i}} \right)BeC{l_2}$$ is monomer in solid state ; $$\left( {{\text{ii}}} \right)BeC{l_2}$$ is linear polymer in vapour phase
D.
$$\left( {\text{i}} \right)BeC{l_2}$$ is linear monomer ; $$\left( {{\text{ii}}} \right)BeC{l_2}$$ is three dimensional dimer.
Answer :
$$\left( {\text{i}} \right)BeC{l_2}$$ is a dimer in vapour phase ; $$\left( {{\text{ii}}} \right)BeC{l_2}$$ is chain structure in solid state
Solution :
(i) is a dimer $$BeC{l_2}$$ in vapour phase.
(ii) is a chain structure of $$BeC{l_2}$$ in solid state.
(i) is a dimer $$BeC{l_2}$$ in vapour phase.
(ii) is a chain structure of $$BeC{l_2}$$ in solid state.