Question
The enolic form of acetone contains
A.
9 sigma bonds, 1 pi - bond and 2 lone pairs
B.
8 sigma bonds, 2 pi - bonds and 2 lone pairs
C.
10 sigma bonds, 1 pi-bond and 1 lone pair
D.
9 sigma bonds, 2 pi - bonds and 1 lone pair
Answer :
9 sigma bonds, 1 pi - bond and 2 lone pairs
Solution :
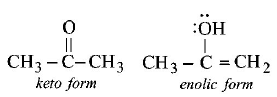
No. of $$\sigma $$ bonds in enolic form: 3 + 1 + 1 + 1 + 1 + 2 = 9
No. of $$\pi $$ bonds in enolic form : 1
No. of lone pairs of electrons in enolic form = 2
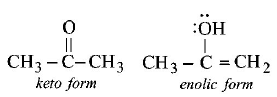
No. of $$\sigma $$ bonds in enolic form: 3 + 1 + 1 + 1 + 1 + 2 = 9
No. of $$\pi $$ bonds in enolic form : 1
No. of lone pairs of electrons in enolic form = 2