Question
The correct order of reactivity towards the electrophilic substitution of the compounds aniline (I) benzene (II) and nitrobenzene (III) is
A.
II < III > I
B.
I > II > III
C.
III > II > I
D.
II > III > I
Answer :
I > II > III
Solution :
In aniline $$ - N{H_2}$$ group is attached with benzene ring. $$ - N{H_2}$$ group shows $$+M$$ - effect. So, it activates the benzene ring. Hence, rate of electrophilic substitution is increased due to increase in the electron density at $$o/p$$ - position. In case of nitrobenzene, $$\left( { - N{O_2}} \right) - M$$ -effect deactivates the benzene ring. So in nitrobenzene, rate of electrophilic substitution is lower than benzene. Hence, order of $${S_E}$$ reaction is
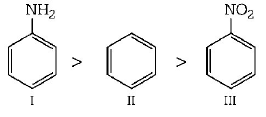
In aniline $$ - N{H_2}$$ group is attached with benzene ring. $$ - N{H_2}$$ group shows $$+M$$ - effect. So, it activates the benzene ring. Hence, rate of electrophilic substitution is increased due to increase in the electron density at $$o/p$$ - position. In case of nitrobenzene, $$\left( { - N{O_2}} \right) - M$$ -effect deactivates the benzene ring. So in nitrobenzene, rate of electrophilic substitution is lower than benzene. Hence, order of $${S_E}$$ reaction is
