Question
The correct order of magnetic moments ( spin only values in $$B.M.$$ ) among is
The correct order of magnetic moments ( spin only values in $$B.M.$$ ) among is
$${\text{(}}\,{\text{Atomic nos}}{\text{.}}\,\,:Mn = 25,Fe = 26,Co = 27\,)$$
A.
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }} > {\left[ {MnC{l_4}} \right]^{2 - }} > {\left[ {CoC{l_4}} \right]^{2 - }}$$
B.
$${\left[ {MnC{l_4}} \right]^{2 - }} > {\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }} > {\left[ {CoC{l_4}} \right]^{2 - }}$$
C.
$${\left[ {MnC{l_4}} \right]^{2 - }} > {\left[ {CoC{l_4}} \right]^{2 - }} > {\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}$$
D.
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }} > {\left[ {CoC{l_4}} \right]^{2 - }} > {\left[ {MnC{l_4}} \right]^{2 - }}$$
Answer :
$${\left[ {MnC{l_4}} \right]^{2 - }} > {\left[ {CoC{l_4}} \right]^{2 - }} > {\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}$$
Solution :
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }} \to $$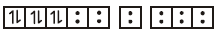
no of unpaired electron $$= 0$$
$${\left[ {MnC{l_4}} \right]^{2 - }} \to $$
no of unpaired electron $$= 5$$
$${\left[ {CoC{l_4}} \right]^{2 - }} \to $$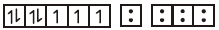
no of unpaired electron $$= 3$$
The greater the number of unpaired electrons, greater the magnitude of magnetic moment. Hence the correct order will be $${\left[ {MnC{l_4}} \right]^{2 - }} > {\left[ {CoC{l_4}} \right]^{2 - }} > {\left[ {Fe{{\left( {Cn} \right)}_6}} \right]^{4 - }}$$
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }} \to $$

no of unpaired electron $$= 0$$
$${\left[ {MnC{l_4}} \right]^{2 - }} \to $$

no of unpaired electron $$= 5$$
$${\left[ {CoC{l_4}} \right]^{2 - }} \to $$
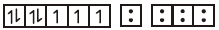
no of unpaired electron $$= 3$$
The greater the number of unpaired electrons, greater the magnitude of magnetic moment. Hence the correct order will be $${\left[ {MnC{l_4}} \right]^{2 - }} > {\left[ {CoC{l_4}} \right]^{2 - }} > {\left[ {Fe{{\left( {Cn} \right)}_6}} \right]^{4 - }}$$