Question
The correct order of increasing $$C - O$$ bond length of $$CO,C{O_2}$$ and $$CO_3^{2 - }$$ is :
A.
$$CO_3^{2 - } < C{O_2} < CO$$
B.
$$C{O_2} < CO_3^{2 - } < CO$$
C.
$$CO < CO_3^{2 - } < C{O_2}$$
D.
$$CO < C{O_2} < CO_3^{2 - }$$
Answer :
$$CO < C{O_2} < CO_3^{2 - }$$
Solution :
Structures of $$CO,C{O_2}$$ and $$CO_3^{2 - }$$ are :
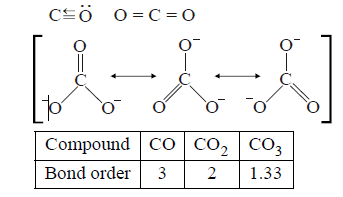
$${\text{Bond order}} \propto \frac{1}{{{\text{Bond length}}}}$$
Hence, the decreasing $$\left( {C - O} \right)$$ bond length is : $$CO < C{O_2} < CO_3^{2 - }$$
Structures of $$CO,C{O_2}$$ and $$CO_3^{2 - }$$ are :

$${\text{Bond order}} \propto \frac{1}{{{\text{Bond length}}}}$$
Hence, the decreasing $$\left( {C - O} \right)$$ bond length is : $$CO < C{O_2} < CO_3^{2 - }$$