Question
The correct order of increasing bond angles in the following species is
A.
$$C{l_2}O < Cl{O_2} < ClO_2^ - $$
B.
$$Cl{O_2} < C{l_2}O < ClO_2^ - $$
C.
$$C{l_2}O < ClO_2^ - < Cl{O_2}$$
D.
$$ClO_2^ - < C{l_2}O < Cl{O_2}$$
Answer :
$$ClO_2^ - < C{l_2}O < Cl{O_2}$$
Solution :
According to $$VSEPR$$ theory repulsion order
$$lp - lp > lp - bp > bp - bp$$
Key Idea As the number of lone pairs of electrons increases, bond angle decreases due to repulsion between $$lp - lp.$$ Moreover, as the electronegativity of central atom decreases, bond angle decreases.
Hence, the order of bond angle is
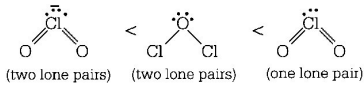
( $$Cl$$ is less electronegative as compared to $$O$$ ).
According to $$VSEPR$$ theory repulsion order
$$lp - lp > lp - bp > bp - bp$$
Key Idea As the number of lone pairs of electrons increases, bond angle decreases due to repulsion between $$lp - lp.$$ Moreover, as the electronegativity of central atom decreases, bond angle decreases.
Hence, the order of bond angle is

( $$Cl$$ is less electronegative as compared to $$O$$ ).