Question
The correct order of acid strength of the following carboxylic
acid is
The correct order of acid strength of the following carboxylic
acid is

A.
I > II > III > IV
B.
II > I > IV > III
C.
I > III > II > IV
D.
III > II > I > IV
Answer :
I > II > III > IV
Solution :
Acidic strength depends upon the stability of anion formed of stability of anion formed is high, its acidic strength will also be high. Order of stability of formed anion :
$$HC \equiv CO{O^ - } > {H_2}C = CHCO{O^ - } > {C_6}{H_4}\left( {OMe} \right)CO{O^ - } > {H_3}CC{H_2}CO{O^ - }$$
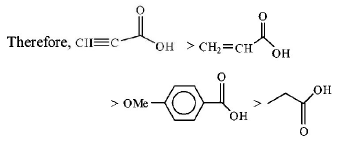
Acidic strength depends upon the stability of anion formed of stability of anion formed is high, its acidic strength will also be high. Order of stability of formed anion :
$$HC \equiv CO{O^ - } > {H_2}C = CHCO{O^ - } > {C_6}{H_4}\left( {OMe} \right)CO{O^ - } > {H_3}CC{H_2}CO{O^ - }$$
