Question
The correct geometry and hybridisation for $$Xe{F_4}$$ are
A.
octahedral, $$s{p^3}{d^2}$$
B.
trigonal bipyramidal, $$s{p^3}d$$
C.
planar triangle, $$s{p^3}{d^3}$$
D.
square planar, $$s{p^3}{d^2}$$
Answer :
octahedral, $$s{p^3}{d^2}$$
Solution :
Key Idea Geometry is determined by electron pair arrangement whereas shape is determined by arrangement of atoms around the centre atom.
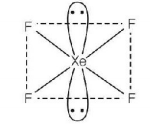
Geometry — octahedral, Hybridisation — $$s{p^3}{d^2}$$
Thus, option (A) is correct.
Key Idea Geometry is determined by electron pair arrangement whereas shape is determined by arrangement of atoms around the centre atom.

Geometry — octahedral, Hybridisation — $$s{p^3}{d^2}$$
Thus, option (A) is correct.