Question
The boiling point of $$p$$ - nitrophenol is higher than that of $$o$$ - nitrophenol because
A.
$$N{O_2}$$ group at $$p$$ - position behave in a different way from that at $$o$$ - position
B.
intramolecular hydrogen bending exists in $$p$$ - nitrophenol
C.
there is intermolecular hydrogen bonding in $$p$$ - nitrophenol
D.
$$p$$ - nitrophenol has a higher molecular weight than $$o$$ - nitrophenol
Answer :
there is intermolecular hydrogen bonding in $$p$$ - nitrophenol
Solution :
The boiling point of $$p$$ - nitrophenol is higher than that of $$o$$ - nitrophenol because $$p$$ - nitrophenol have intermolecular hydrogen bonding whereas $$o$$ - nitrophenol have intramolecular $$H$$ - bonding as given below
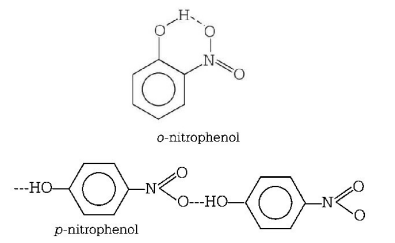
The boiling point of $$p$$ - nitrophenol is higher than that of $$o$$ - nitrophenol because $$p$$ - nitrophenol have intermolecular hydrogen bonding whereas $$o$$ - nitrophenol have intramolecular $$H$$ - bonding as given below
