Question
The activation energy of the reaction, $$A + B \to C + D + 38\,kcal$$ is $$20\,kcal.$$ What would be the activation energy of the following reaction.
The activation energy of the reaction, $$A + B \to C + D + 38\,kcal$$ is $$20\,kcal.$$ What would be the activation energy of the following reaction.
$$C + D \to A + B$$
A.
20$$\,kcal$$
B.
- 20$$\,kcal$$
C.
18$$\,kcal$$
D.
58$$\,kcal$$
Answer :
58$$\,kcal$$
Solution :
$${E_{{a_2}}} = 58$$
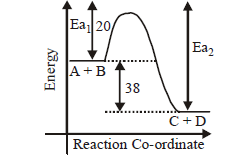
$${E_{{a_2}}} = 58$$
