Question
Pure water freezes at $$273 K$$ and 1 bar. The addition of $$34.5 g$$ of ethanol to $$500 g$$ of water changes the freezing point of
the solution. Use the freezing point depression constant of water as $$2\,K\,kg\,mo{l^{ - 1}}.$$ The figures shown below represent
plots of vapour pressure $$(V.P.)$$ versus temperature $$(T).$$ [ molecular weight of ethanol is $$46\,g\,mo{l^{ - 1}}$$ ] Among the
following, the option representing change in the freezing point is
Pure water freezes at $$273 K$$ and 1 bar. The addition of $$34.5 g$$ of ethanol to $$500 g$$ of water changes the freezing point of
the solution. Use the freezing point depression constant of water as $$2\,K\,kg\,mo{l^{ - 1}}.$$ The figures shown below represent
plots of vapour pressure $$(V.P.)$$ versus temperature $$(T).$$ [ molecular weight of ethanol is $$46\,g\,mo{l^{ - 1}}$$ ] Among the
following, the option representing change in the freezing point is
A.
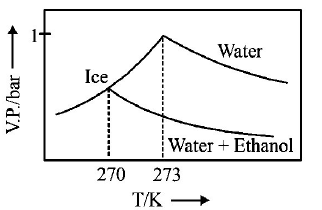

B.
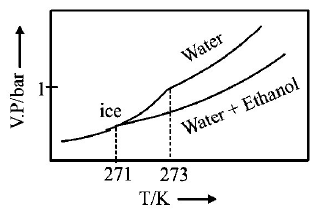

C.
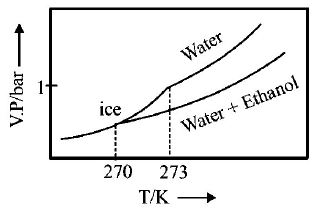

D.
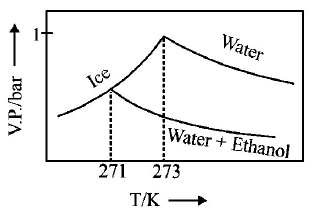

Answer :


Solution :
$$\eqalign{ & {\text{As T increase, V}}{\text{.P}}{\text{. increases}} \cr & \Delta {T_f} = {K_f} \times m\,\left( {m = {\text{molality of solute}}} \right) \cr & 273 - T{'_f} = 2 \times \frac{{34.5 \times 1000}}{{46 \times 500}} \cr & \therefore \,\,T{'_f} = 270\,K \cr} $$
Thus freezing point of solution $$= 270K.$$ Further as T increases, vapour pressure increases. Hence these facts coincide with the curve given in (C).
$$\eqalign{ & {\text{As T increase, V}}{\text{.P}}{\text{. increases}} \cr & \Delta {T_f} = {K_f} \times m\,\left( {m = {\text{molality of solute}}} \right) \cr & 273 - T{'_f} = 2 \times \frac{{34.5 \times 1000}}{{46 \times 500}} \cr & \therefore \,\,T{'_f} = 270\,K \cr} $$
Thus freezing point of solution $$= 270K.$$ Further as T increases, vapour pressure increases. Hence these facts coincide with the curve given in (C).