Question
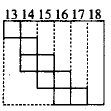
Part of the periodic table showing $$p$$ - block is depicted below. What are
the elements shown in the zig-zag boxes called? What is the nature of
the elements outside this boundary on the right side of the table?
Part of the periodic table showing $$p$$ - block is depicted below. What are
the elements shown in the zig-zag boxes called? What is the nature of
the elements outside this boundary on the right side of the table?
A.
Transition elements, metalloids
B.
Metalloids, non-metals
C.
Metals, non-metals
D.
Non-metals, noble gases
Answer :
Metalloids, non-metals
Solution :
Metalloids are shown by the zig-zag boxes and the elements present on the right side of the boundary are nonmetals. Metals are present on the left side of the periodic table.
Metalloids are shown by the zig-zag boxes and the elements present on the right side of the boundary are nonmetals. Metals are present on the left side of the periodic table.