Question
Ortho-Nitrophenol is less soluble in water than $$p{\text{ - }}$$ and $$m{\text{ - }}$$ Nitrophenols because :
A.
$$o{\text{ - }}$$Nitrophenol is more volatile steam than those of
$$m{\text{ - }}$$ and $$p{\text{ - }}$$isomers.
B.
$$o{\text{ - }}$$Nitrophenol shows intramolecular $$H$$ - bonding
C.
$$o{\text{ - }}$$Nitrophenol shows intermolecular $$H$$ - bonding
D.
Melting point of $$o{\text{ - }}$$Nitrophenol is lower than those of $$m{\text{ - }}$$ and $$p{\text{ - }}$$isomers.
Answer :
$$o{\text{ - }}$$Nitrophenol shows intramolecular $$H$$ - bonding
Solution :
Compounds involved in chelation become non-polar. Consequently such compounds are soluble in nonpolar solvents like ether, benzene etc. and are only sparingly soluble in water whereas meta and para isomers are more soluble in water & less soluble in non-polar solvents.
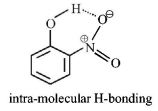
Compounds involved in chelation become non-polar. Consequently such compounds are soluble in nonpolar solvents like ether, benzene etc. and are only sparingly soluble in water whereas meta and para isomers are more soluble in water & less soluble in non-polar solvents.
