Question
“Metals are usually not found as nitrates in their ores”.
“Metals are usually not found as nitrates in their ores”.
Out of the following two ( I and II ) reasons which is/are true for the above observation ?
I. Metal nitrates are highly unstable.
II. Metal nitrates are highly soluble in water.
A.
I and II are true
B.
I and II are false
C.
I is false but II is true
D.
I is true but II is false
Answer :
I is false but II is true
Solution :
Metals are usually not found as nitrates in their ores, because metal nitrates are highly soluble in water. For example, $$KN{O_3}$$ ( salt peter ) would be classified as completely soluble. Thus, $$KN{O_3}$$ could be expected to dissociate completely in aqueous solution to give $${K^ + }$$ and $$NO_3^ - \,ions.$$
$$KN{O_3} \rightleftharpoons {K^ + }\left( {aq} \right) + NO_3^ - \left( {aq} \right)$$
The nitrate anion has three equivalent oxygen surrounding a central nitrogen atom. This tends to spread the single negative charge and make it easier for water ( using hydrogen bonds ) to separate the ions in solution.
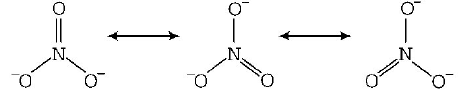
Metals are usually not found as nitrates in their ores, because metal nitrates are highly soluble in water. For example, $$KN{O_3}$$ ( salt peter ) would be classified as completely soluble. Thus, $$KN{O_3}$$ could be expected to dissociate completely in aqueous solution to give $${K^ + }$$ and $$NO_3^ - \,ions.$$
$$KN{O_3} \rightleftharpoons {K^ + }\left( {aq} \right) + NO_3^ - \left( {aq} \right)$$
The nitrate anion has three equivalent oxygen surrounding a central nitrogen atom. This tends to spread the single negative charge and make it easier for water ( using hydrogen bonds ) to separate the ions in solution.
